Casino Bizzo es un casino online español que ofrece una amplia variedad de juegos de casino. El casino en línea se encuentra en funcionamiento desde 2020 y ofrece una gran cantidad de bonos y promociones a sus jugadores. Sin embargo, algunos usuarios se han quejado de que el casino no es muy seguro y confiable.
¿El casino Bizzo es seguro?
Casino Bizzo es un casino online seguro y confiable. El casino cumple con todas las normas y regulaciones de la Unión Europea y está regulado por la Comisión de Juego del Reino Unido. El casino en línea utiliza la tecnología de cifrado SSL para proteger la información personal y financiera de sus jugadores. Además, el casino ofrece una protección contra el fraude y el lavado de dinero.
¿El casino Bizzo es legítimo?
 Sí, Casino Bizzo es un casino online legítimo y seguro. El casino cumple con todas las normas y regulaciones de la Unión Europea y está regulado por la Comisión de Juego del Reino Unido. El casino en línea utiliza la tecnología de cifrado SSL para proteger la información personal y financiera de sus jugadores. Además, el casino ofrece una protección contra el fraude y el lavado de dinero.
Sí, Casino Bizzo es un casino online legítimo y seguro. El casino cumple con todas las normas y regulaciones de la Unión Europea y está regulado por la Comisión de Juego del Reino Unido. El casino en línea utiliza la tecnología de cifrado SSL para proteger la información personal y financiera de sus jugadores. Además, el casino ofrece una protección contra el fraude y el lavado de dinero.
¿Cómo puedo saber si el casino Bizzo es seguro?
Hay varias formas en que puedes comprobar si Casino Bizzo es seguro y confiable. En primer lugar, puedes verificar si el casino está regulado y licenciado por un organismo de juego reconocido. En segundo lugar, puedes leer las opiniones y comentarios de otros jugadores para saber si el casino es seguro y confiable. En tercer lugar, puedes visitar el sitio web del casino y verificar si utiliza la tecnología de cifrado SSL para proteger la información personal y financiera de sus jugadores.
¿Cómo puedo depositar dinero en el casino Bizzo?
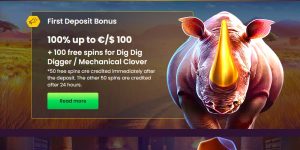
El casino Bizzo ofrece a sus jugadores una amplia variedad de métodos de pago para depositar y retirar dinero. Los jugadores pueden depositar dinero en el casino a través de tarjetas de crédito/débito, transferencias bancarias, e-wallets y criptomonedas. El casino no cobra ninguna tarifa por los depósitos y los retiros se procesan de forma rápida y segura.
¿Cómo puedo contactar al casino Bizzo?
Hay varias formas en que puedes contactar al casino Bizzo si tienes alguna duda o consulta. El casino ofrece un servicio de atención al cliente las 24 horas del día, los 7 días de la semana. Puedes ponerte en contacto con el casino a través de teléfono, correo electrónico o chat en vivo. Además, el casino tiene una sección de preguntas frecuentes donde puedes encontrar respuestas a las dudas más comunes.
Descubra por qué un equipo de expertos no recomienda el nuevo casino en línea Bizzo este año.
También te pueden interesar estos casinos online:
¿Cómo puedo abrir una cuenta en el casino Bizzo?
 Abriendo una cuenta en el casino Bizzo es muy fácil y seguro. El proceso de registro es muy simple y rápido. Solo necesitas proporcionar algunos datos básicos como tu nombre, dirección de correo electrónico, número de teléfono y fecha de nacimiento. Una vez que hayas completado el registro, ya podrás disfrutar de todos los juegos y servicios del casino.
Abriendo una cuenta en el casino Bizzo es muy fácil y seguro. El proceso de registro es muy simple y rápido. Solo necesitas proporcionar algunos datos básicos como tu nombre, dirección de correo electrónico, número de teléfono y fecha de nacimiento. Una vez que hayas completado el registro, ya podrás disfrutar de todos los juegos y servicios del casino.
Ventajas de Casino Bizzo
- Gran variedad de juegos de casino
- Bonos y promociones atractivos
- Seguro y confiable
- Cumple con todas las normas y regulaciones
- Ofrece una protección contra el fraude y el lavado de dinero
- Amplia variedad de métodos de pago
- Servicio de atención al cliente las 24 horas del día
- Proceso de registro muy simple y rápido
Desventajas de Casino Bizzo
- Algunos usuarios se han quejado de que el casino no es muy seguro y confiable


